Identifying Potential Nanoscale Sources of Decoherence in Nb Superconducting Qubits
Insights regarding defects in the surface oxide help inform new methods for improving qubit coherence
The Science:
Secondary ion mass spectrometry and advanced electron microscopy was used to conduct a detailed assessment of the surface oxide that forms in ambient conditions for Nb transmon qubit devices. We observe that this oxide exhibits a varying stoichiometry and by mapping the relative crystallinity, we find that highly disordered regions are more likely to contain oxygen vacancies and exhibit weaker bonds between the niobium and oxygen atoms.
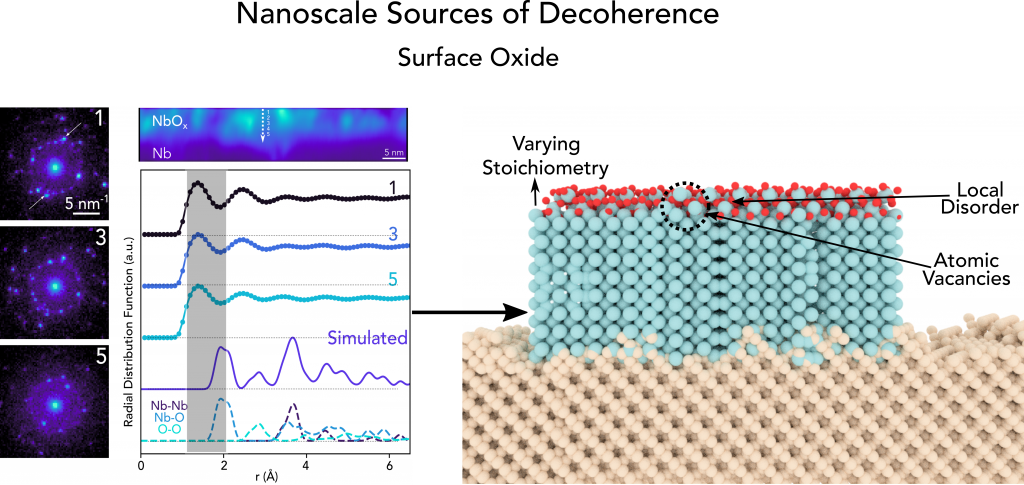
(Right) Overview of inhomogeneities observed in surface oxide of Niobium
The Impact:
These results suggest that oxygen vacancies in the oxide at the surface of Nb likely serve as a decoherence mechanism in quantum systems. This insight allows us to focus efforts on designing and developing new fabrication processes to mitigate the formation of such defects to boost qubit coherence times.
Summary:
Superconducting thin films of niobium have been extensively employed in transmon qubit architectures. Although these architectures have demonstrated improvements in recent years, further improvements in performance through materials engineering will aid in large-scale deployment. Here, we use information retrieved from secondary ion mass spectrometry and electron microscopy to conduct a detailed assessment of the surface oxide that forms in ambient conditions for transmon test qubit devices patterned from a niobium film. We observe that this oxide exhibits a varying stoichiometry with NbO and NbO2 found closer to the niobium film and Nb2O5 found closer to the surface.
In terms of structural analysis, we find that the Nb2O5 region is semicrystalline in nature and exhibits randomly oriented grains on the order of 1-2 nm corresponding to monoclinic N-Nb2O5 that are dispersed throughout an amorphous matrix. Using fluctuation electron microscopy, we are able to map the relative crystallinity in the Nb2O5 region with nanometer spatial resolution. The main outcome of this work is that we observe that the highly disordered regions are more likely to contain oxygen vacancies and exhibit weaker bonds between the niobium and oxygen atoms. Based on these findings, we expect that oxygen vacancies likely serve as a decoherence mechanism in these qubits.
Contact:
Akshay Murthy – amurthy@fnal.gov
Focus Area:
Materials for 2D and 3D Quantum Devices
Institutions:
Fermi National Accelerator Laboratory, Northwestern University, Ames National Laboratory, Rigetti Computing
Citation:
Murthy, Akshay A.; Masih Das, Paul; Ribet, Stephanie M.; Kopas, Cameron; Lee, Jaeyel; Reagor, Matthew J.; Zhou, Lin; Kramer, Matthew J.; Hersam, Mark C.; Checchin, Mattia; Grassellino, Anna; Reis, Roberto dos; Dravid, Vinayak P.; Romanenko, Alexander; Developing a Chemical and Structural Understanding of the Surface Oxide in a Niobium Superconducting Qubit. ACS Nano, 16 (10), 2022, 17257–17262.
https://doi.org/10.1021/acsnano.2c07913
Funding Acknowledgement:
This material is based upon work supported by the U.S. Department of Energy, Office of Science, National Quantum Information Science Research Centers, Superconducting Quantum Materials and Systems Center (SQMS) under the contract No. DE-AC02-07CH11359. Fermilab is operated by the Fermi Research Alliance, LLC under contract No. DE-AC02-07CH11359 with the United States Department of Energy. This work made use of the EPIC facility of Northwestern University’s NUANCE Center, which received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205); the MRSEC program (NSF DMR-1720139) at the Materials Research Center; the International Institute for Nanotechnology (IIN); the Keck Foundation; and the State of Illinois, through the IIN.
